There are a few main ways of preparing aniline:
Reduction of the Nitro Group
We can add a nitro group to benzene or its derivative via a standard nitration using HNO3 and H2SO4, and then reduce the nitro group using Zn, Sn, or Fe with HCl, Pd over activated carbon, etc.
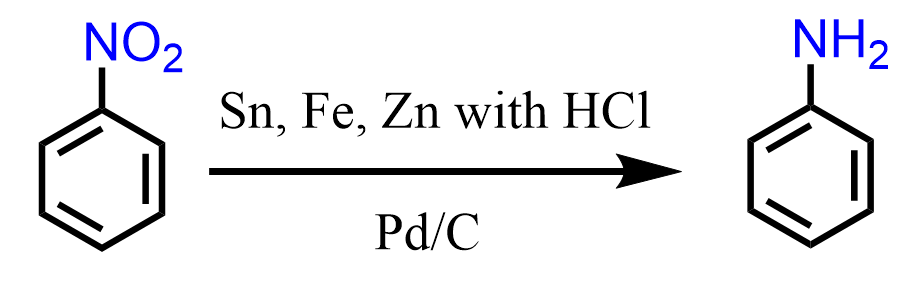
Nucleophilic aromatic substitutions work pretty well for converting aryl halides to aniline derivatives using sodium amide (NaNH2 in ammonia):
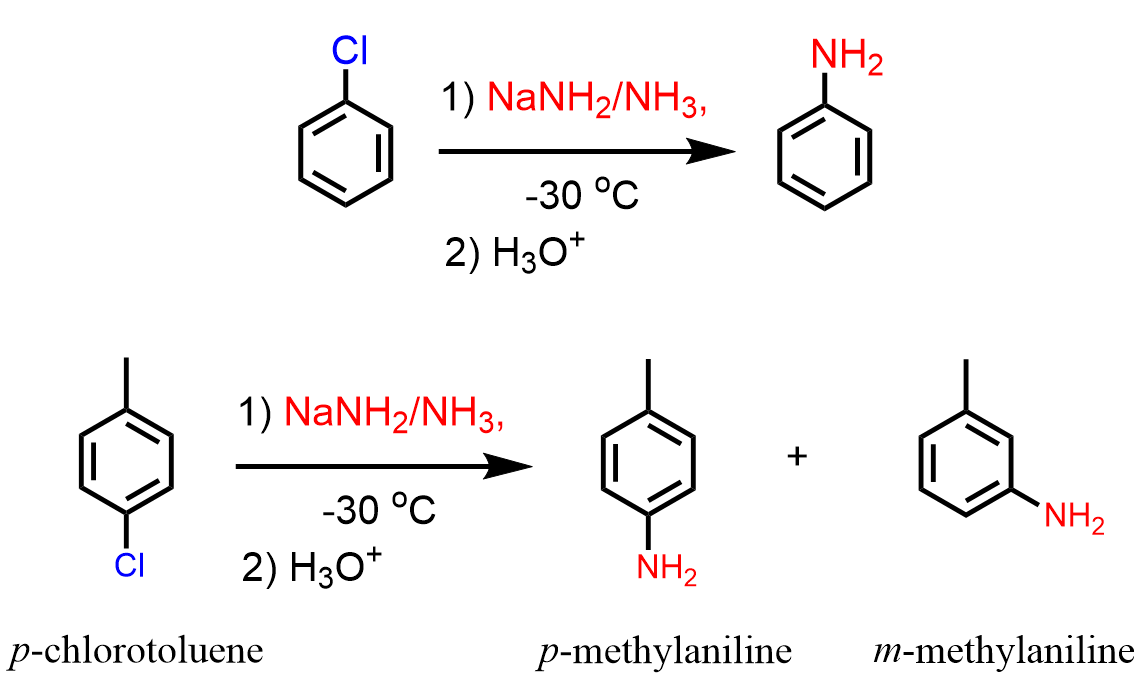
Check the linked article for more details about the mechanism and radiochemistry of these reactions.
Molecular Rearrangements – Hofmann, Beckman
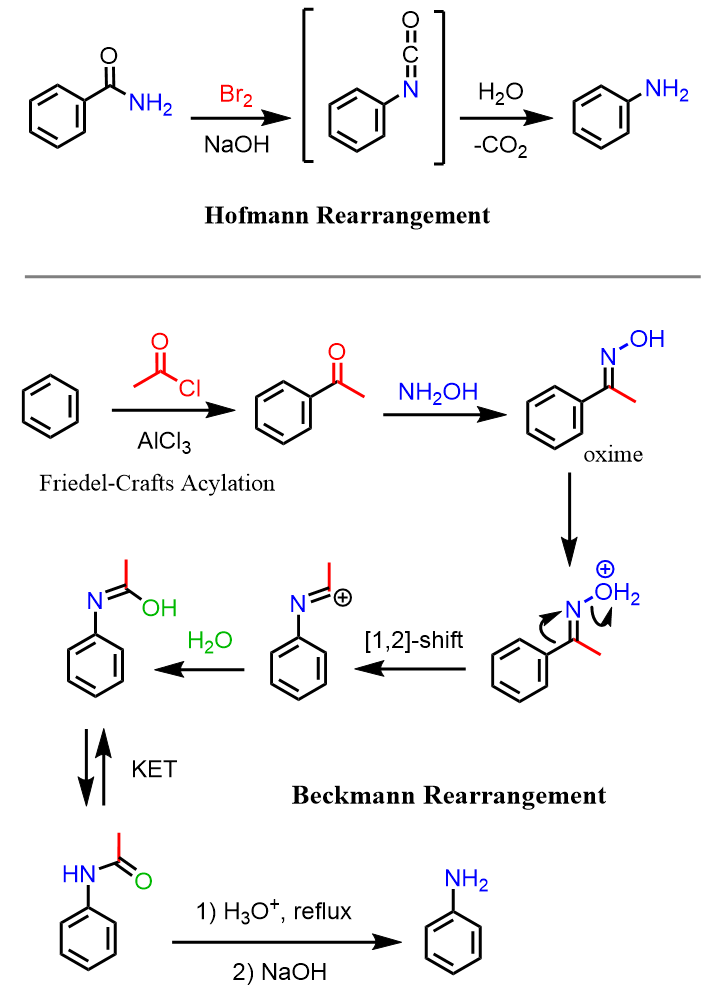
Another strategy for preparing aniline is the use of some rearrangement reactions, such as the Hofmann and Beckman reactions:
Let’s compare the base strength of cyclohexylamine and aniline and state from the beginning that the former is a stronger base. So, you can take a moment to try finding an explanation for this observation:
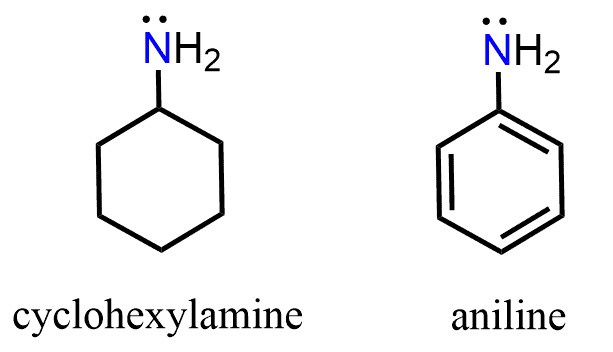
Keep in mind that the base strength of the amine will increase with its readiness to donate the electron pair. Now, the lone pair on the nitrogen in ethyl amine is “not doing anything” other than just sitting there, i.e., it is localized. The one in aniline, on the other hand, is next to an aromatic ring, which is a conjugated system, and if this lone pair can be part of the conjugate system, we can predict that it is not going to be as ready to accept a proton. And, in fact, the lone pair of nitrogen in aniline is delocalized on the benzene ring, and that is why aniline is less basic than ethyl amine or most alkyl amines for that comparison.
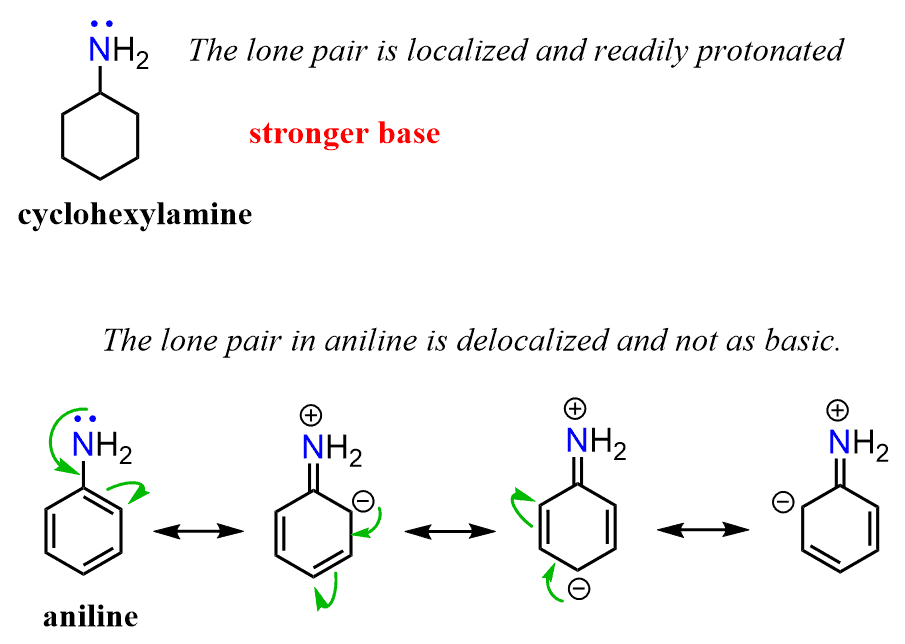
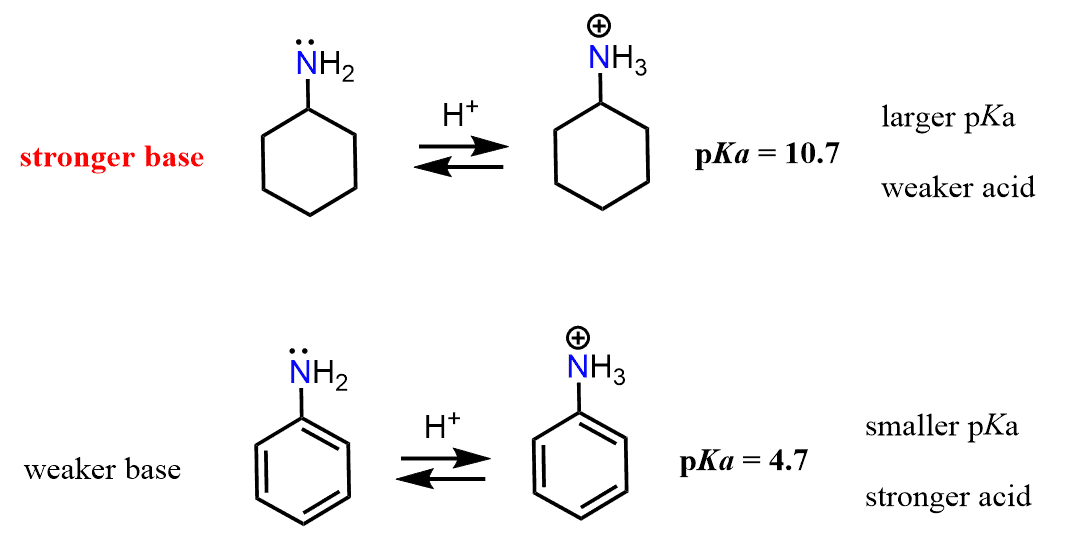
And here as well, the pKa values support this reasoning. Because the pKa of CH3CH2NH3+ is higher than the pKa of C6H5NH3+, CH3CH2NH2 is a stronger base than C6H5NH2.
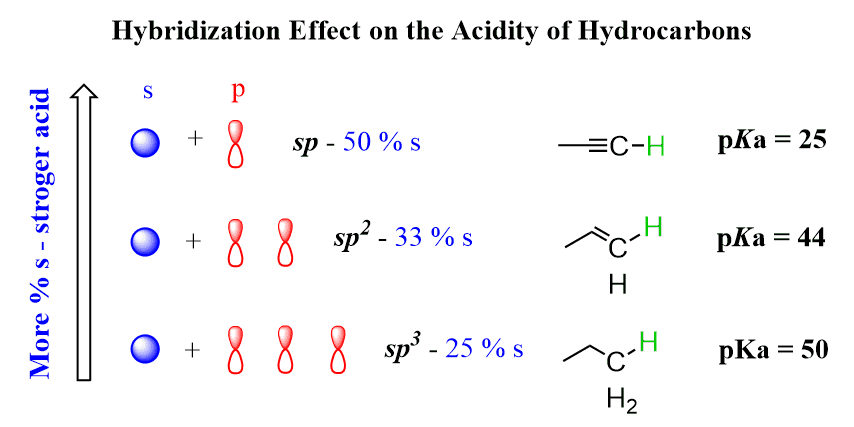
Another factor contributing to the decreased basicity of aromatic amines is the hybridization of the carbon atoms in the aromatic ring. These are sp2 hybridized and sp2 hybridized carbon atoms are more electronegative than sp3 carbons because they have more s character.
As a result, they have an electron-withdrawing inductive effect while the aliphatic carbons are electron donors. In general, the more s character, the more electronegative the atom is. These are the percentages of the s orbital (s-character) in each hybrid orbital:
The relationship of the base strength of aniline and cyclohexyl amine is analogous to comparing the basicity of phenoxide and alkoxide ions. Phenoxide ions are less basic than alkoxide ions for the same reasons mentioned above.
Summarizing this comparison, keep in mind that aromatic amines are considerably weaker bases than aliphatic amines.
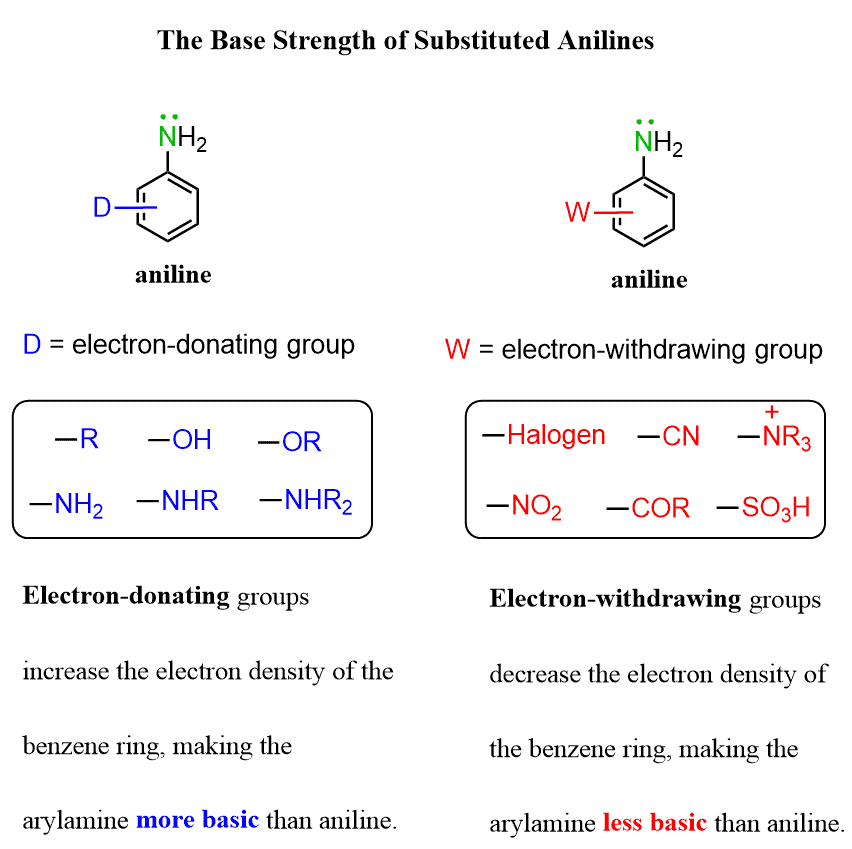
What about the basicity of substituted anilines?
Whether a substituted aniline is more or less basic than aniline depends on the nature of the substituent.
Electron-donating groups increase the electron density of the benzene ring, making the arylamine more basic than aniline.
Electron-withdrawing groups decrease the electron density of the benzene ring, making the arylamine less basic than aniline.
The effect of the nature and the orientation of the other group on the basicity of aniline and other aromatic amines is discussed in more detail here, so feel free to check that article.
Just like we have for phenol and its derivatives, you need to remember that the amino group on an aromatic ring is that it is a strong activator and thus, an ortho, para director.
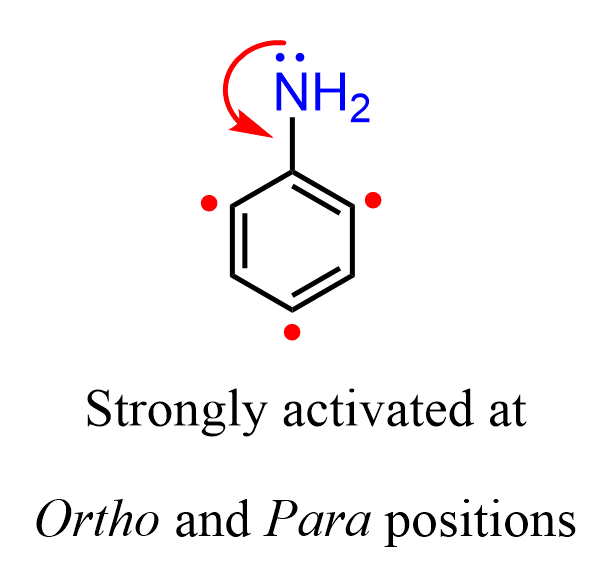
All the electrophilic aromatic substitution reactions are faster when oxygen or nitrogen is connected to the aromatic ring. Now, you may wonder why it is the case if they are more electronegative than carbon and should pull the electron density from the ring and thus making it less reactive. Recall that the reason for such a strong activating power of the oxygen and nitrogen is the resonance stabilization of the cation by the nonbonding electrons, which other activating groups, such as alkyls, do not have:
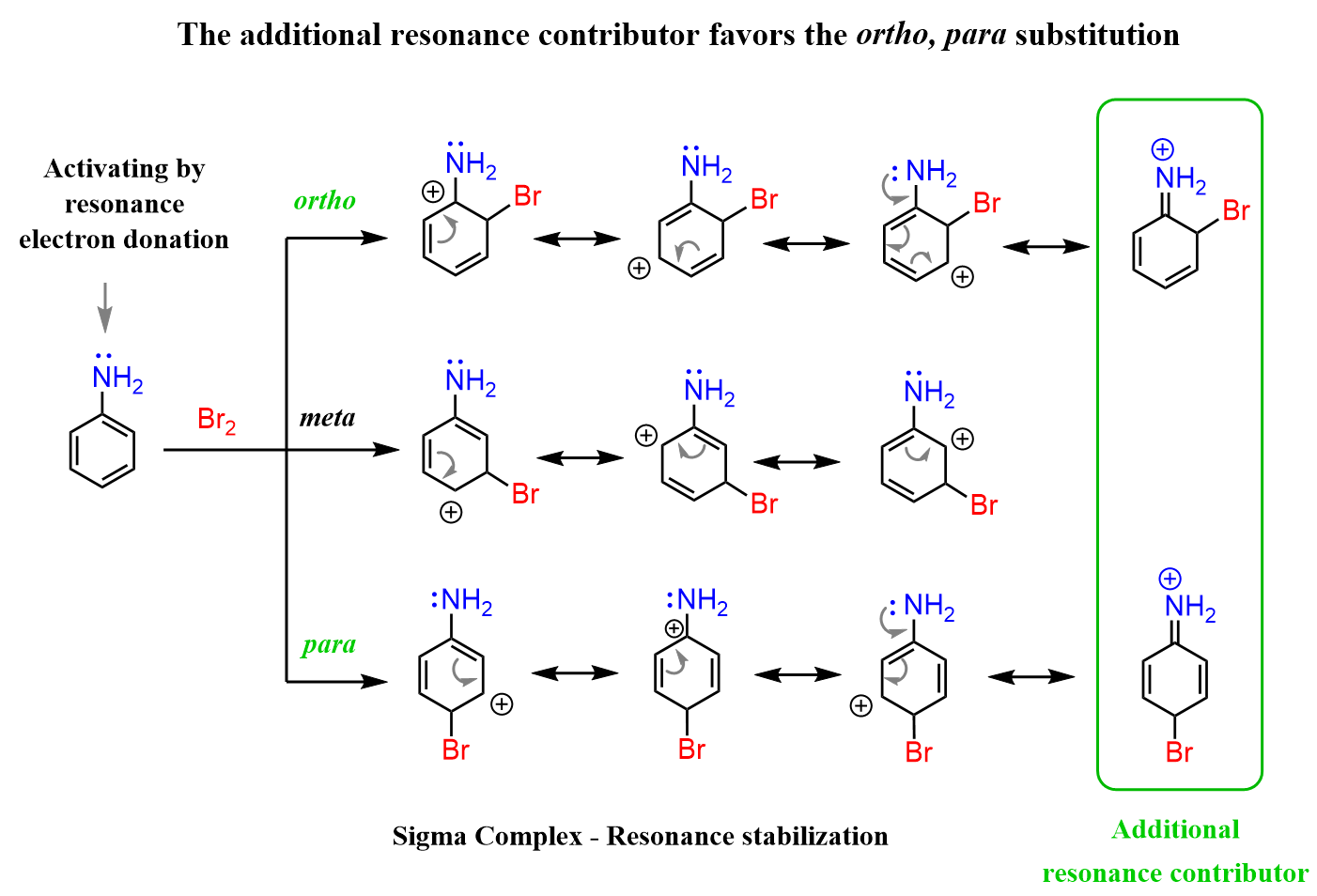
So, even though nitrogen pulls the electron density from the ring via the inductive effect, the +M mesomeric (electron-donating resonance) effect is more profound, which makes it a strongly activating group.
Notice that in the mechanism for the bromination of aniline, we do not show a typical Lewis acid catalyst such as FeBr3. This is again due to the very strong electron-donating effect of the amino group, which makes the aromatic ring extremely electron-rich such that we cannot stop the reaction at monobromination even at lower temperatures, as we saw for phenol:
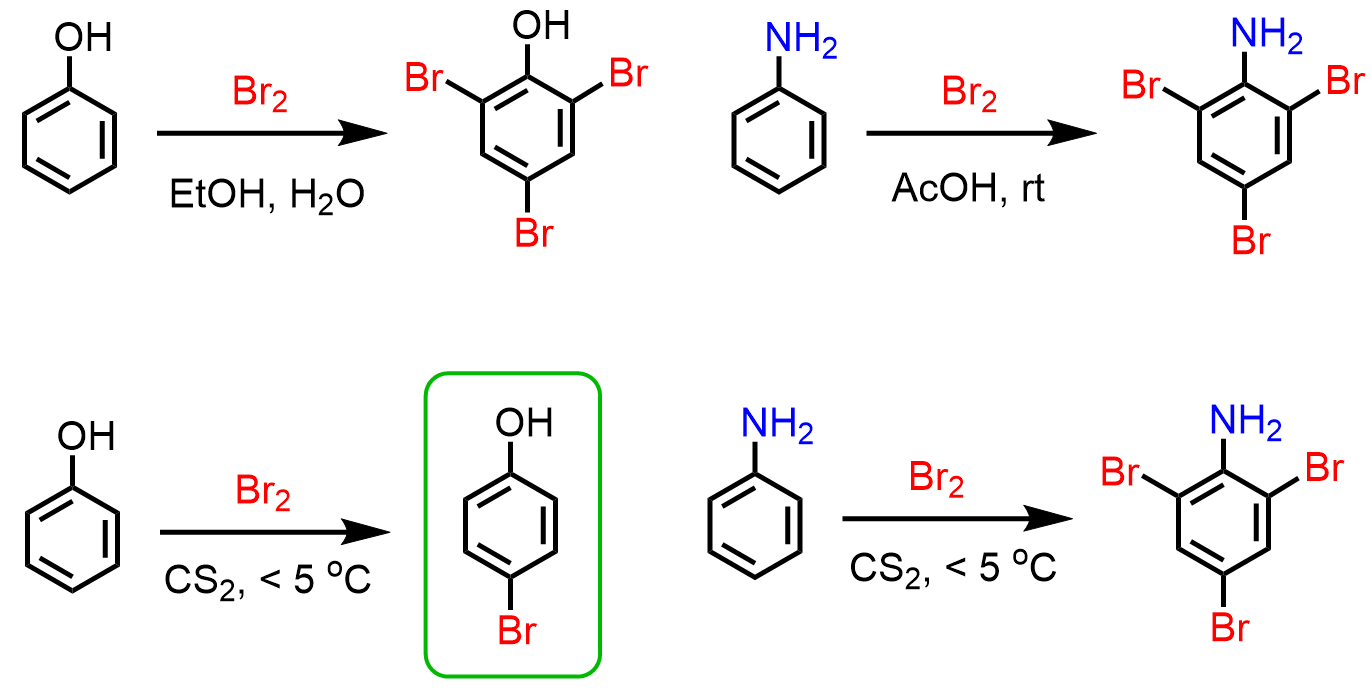
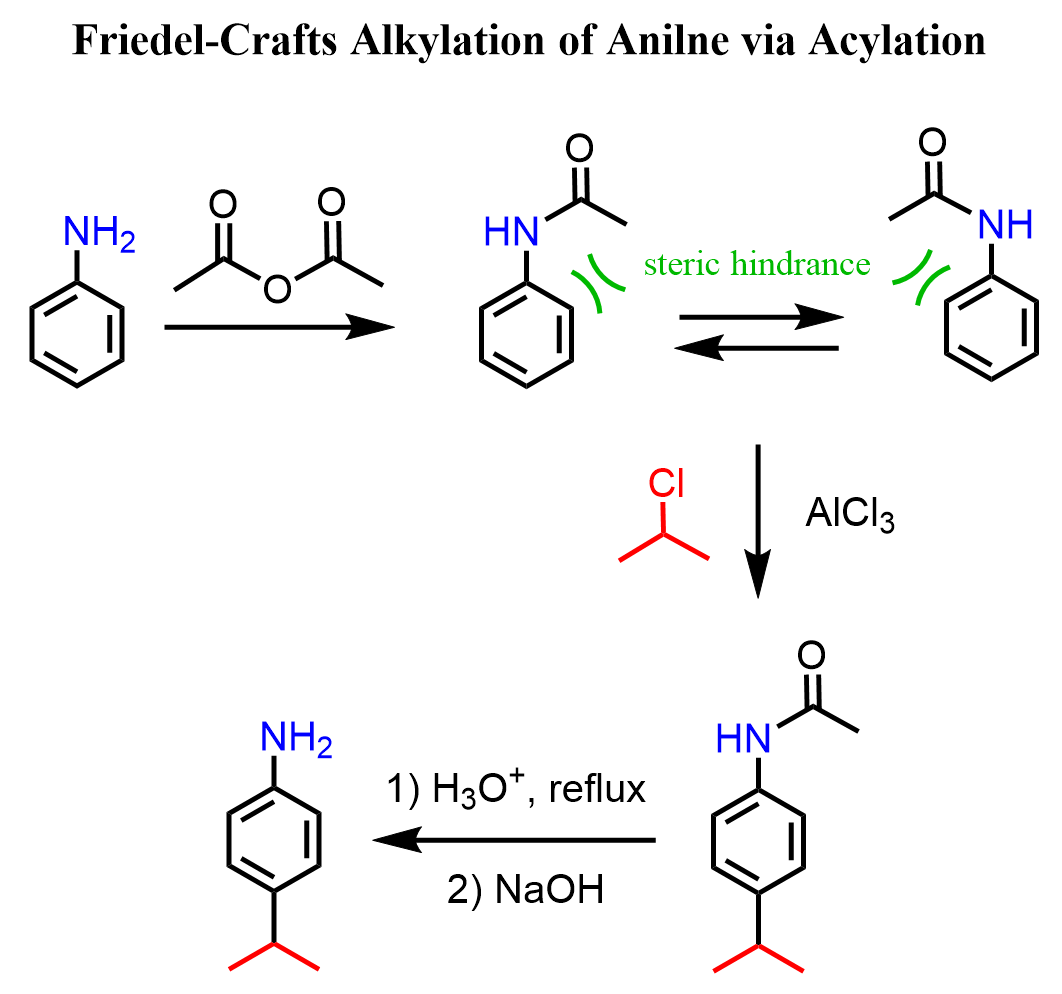
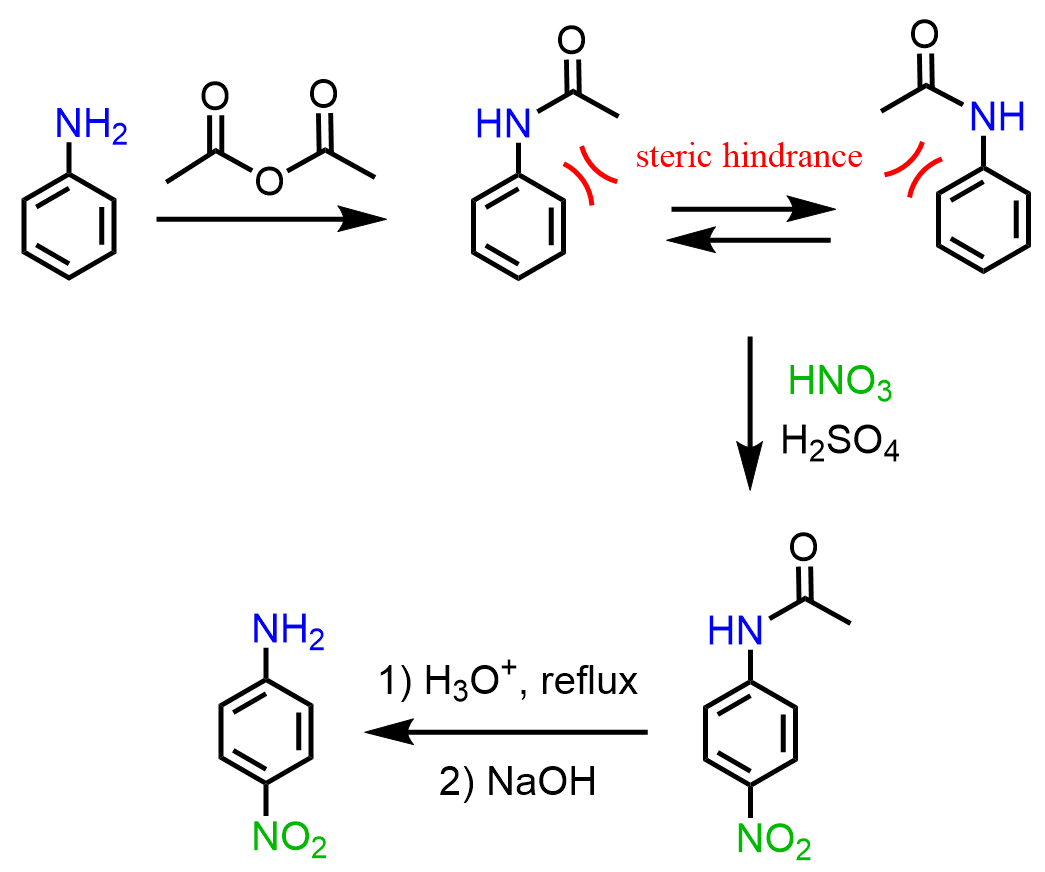
The strategy to stop the trisubstitution and isolate a nonhalogenated aniline is by converting the amino group into an amide via acetylation. The difference between the amino and amide groups is that in the amide, the lone pair of the nitrogen is conjugated with the carbonyl group and is less available to be in resonance with the electrons of the ring:
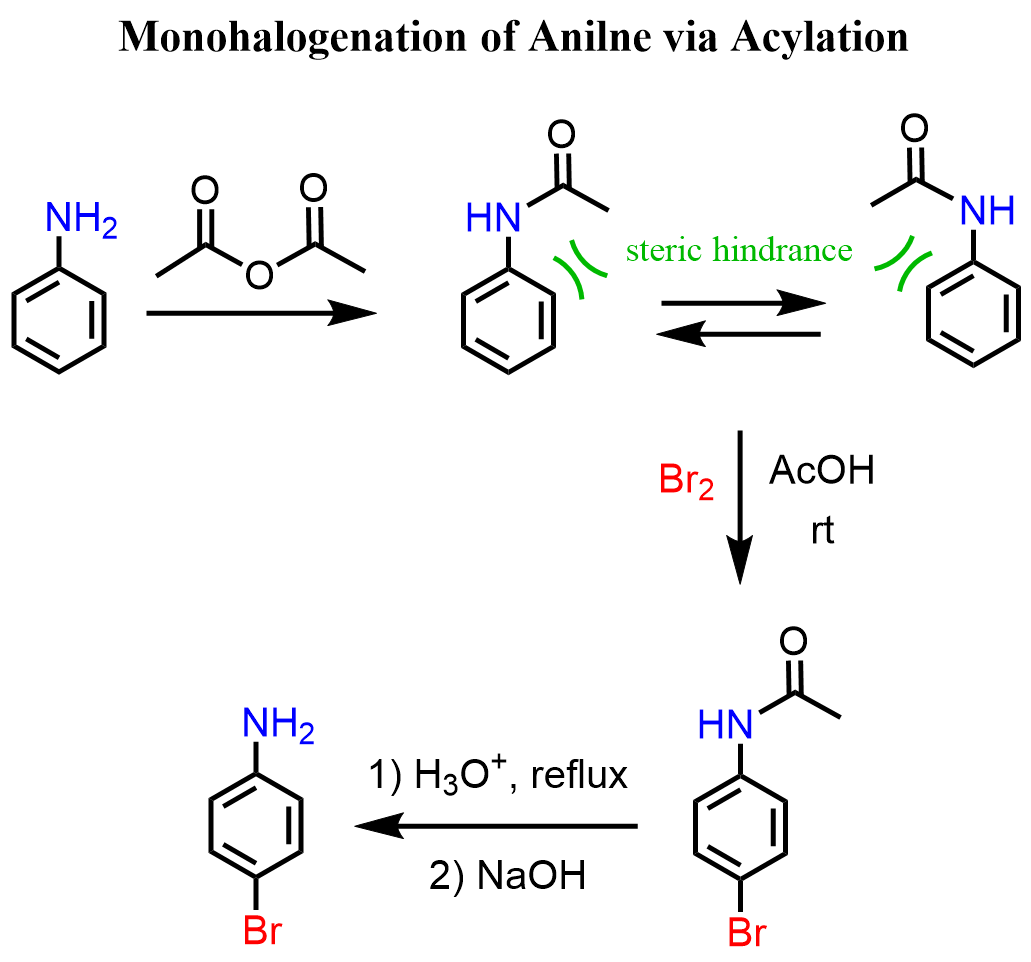
The amide is still an activator and an ortho, para, but not as strong as to cause over-substitution. The steric hindrance by the acetyl group directs the halogenation to the para position.
Even though the amino group activates the benzene ring, and many electrophilic aromatic substitution reactions are faster compared to those with benzene, there are also quite a few that do not work as expected. The reason for this is that aniline is a Lewis base because of the lone pairs on the nitrogen. So, how does the lone pair become an obstacle in the EAS reactions of aniline? Let’s address this by discussing the Friedel-Crafts reactions, which are among the slowest of EAS, and require a Lewis acid catalyst such as AlCl3, FeBr3, etc.
The problem is that when the Lewis acid catalyst is added, it binds to the nitrogen, creating a species where the nitrogen is positively charged:
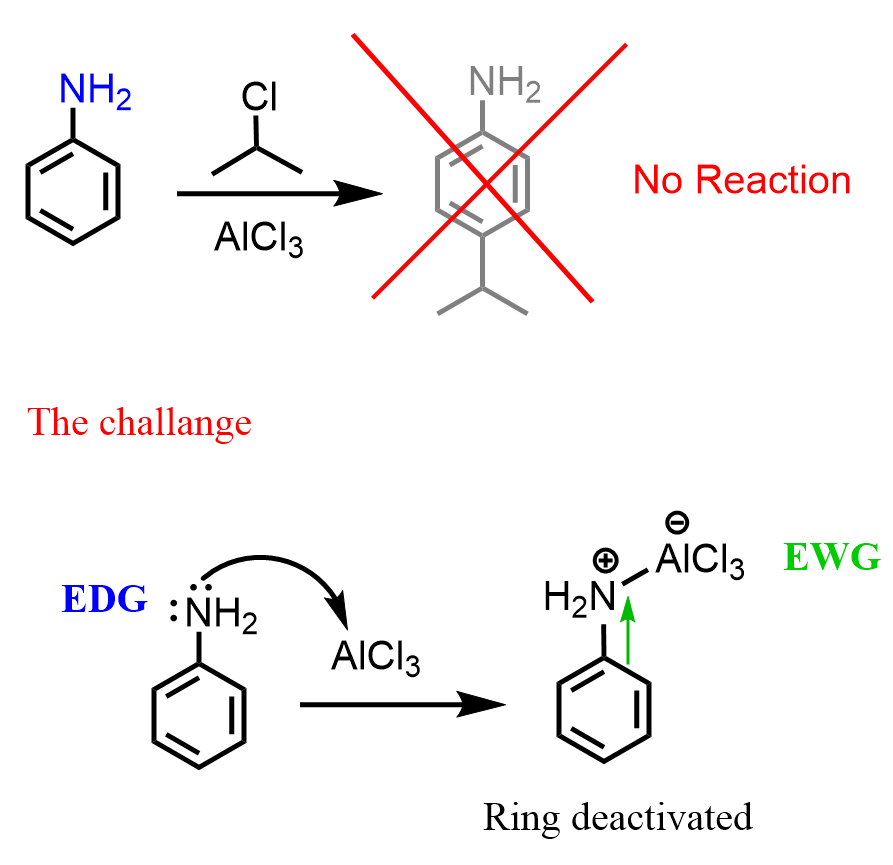
The positively charged nitrogen is no longer a resonance-activator because it pulls the electron density from the ring. Moreover, it is a strangely deactivating substituent on the ring, and Friedel-Crafts reactions, being slow, can no longer occur.
On a side note, the reluctance of deactivated benzene rings towards Friedel-Crafts alkylation and acylation is not the only limitation in electrophilic aromatic substitutions, so check the linked article for more examples and strategies to overcome these limitations.
To overcome the problem with protonating the niteogen, thus turning it into a deactivator and a meta director, the same strategy of acylating the amino group is used:
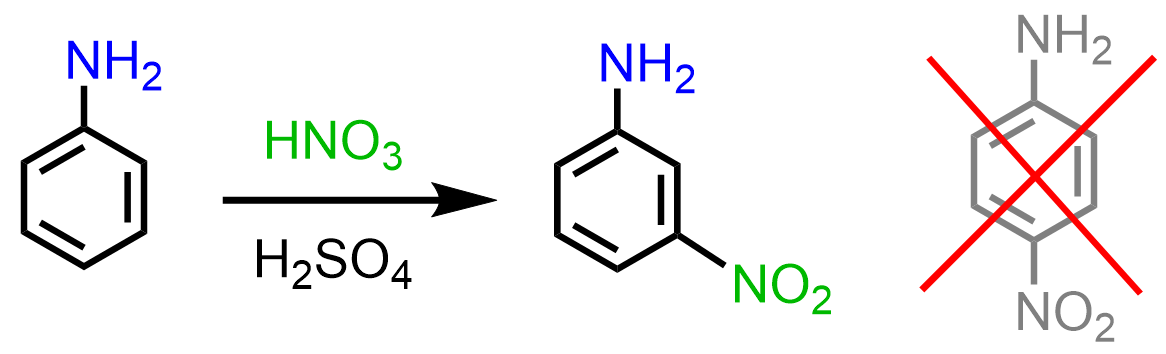
Even though the amino group activates the benzene ring, and some electrophilic aromatic substitution reactions of aniline are faster compared to those with benzene, nitration of aniline does not work as expected. We know that activating groups are ortho, para directors, so expecting ortho, para nitration of aniline might seem a reasonable prediction.
However, it has been shown that this reaction either leads to a mixture of undesirable side products, or, what is interesting, meta-nitroaniline is obtained:
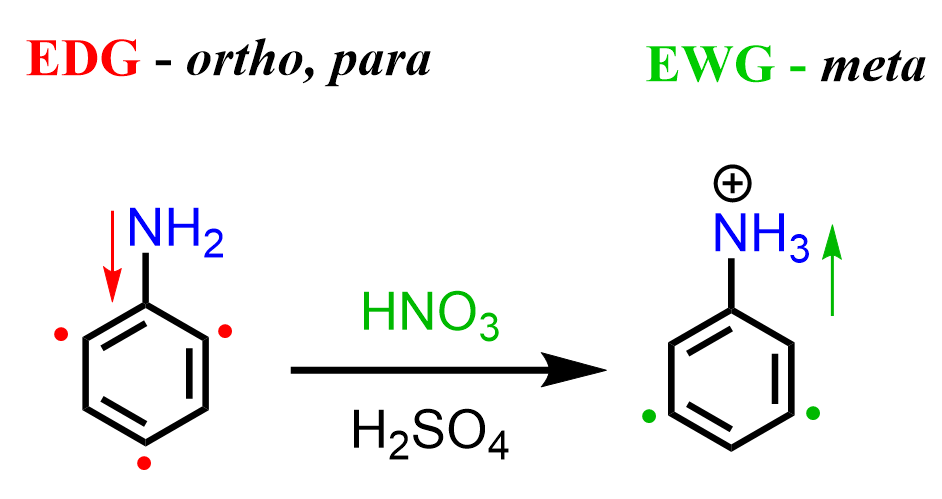
The formation of the unexpected meta product is again due to the basicity of the amino group. While in Friedel Crafts reactions, it acts as a Lewis base; here, we have a regular Bronsted acid-base reaction with HNO3 and H2SO4. Once the amino group is protonated, a strongly deactivating -NH3+ group is formed, which now undergoes a meta EAS:
In this case, as well, the strategy is to convert the amino group to an amide and hydrolyze it back to amine once the nitration is complete.
Interestingly, it was found that, unlike nitration, aniline can be submitted to direct sulfonation using concentrated sulfuric acid at 190 oC, and the para isomer is obtained (Clayden – Organic Chemistry):
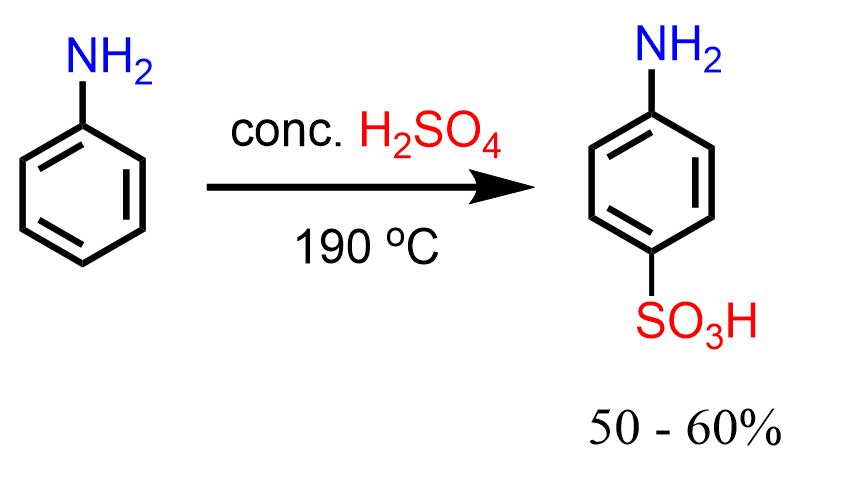
One explanation for this is the superior reactivity of the trace amount of the unprotonated aniline.
Using the Friedel-Crafts acylation, we can prepare aryl ketones by reacting aromatic compounds with an acyl halide in the presence of a Lewis acid catalyst.
To prepare an aldehyde, the Valmeyer-Hack reaction can be used:
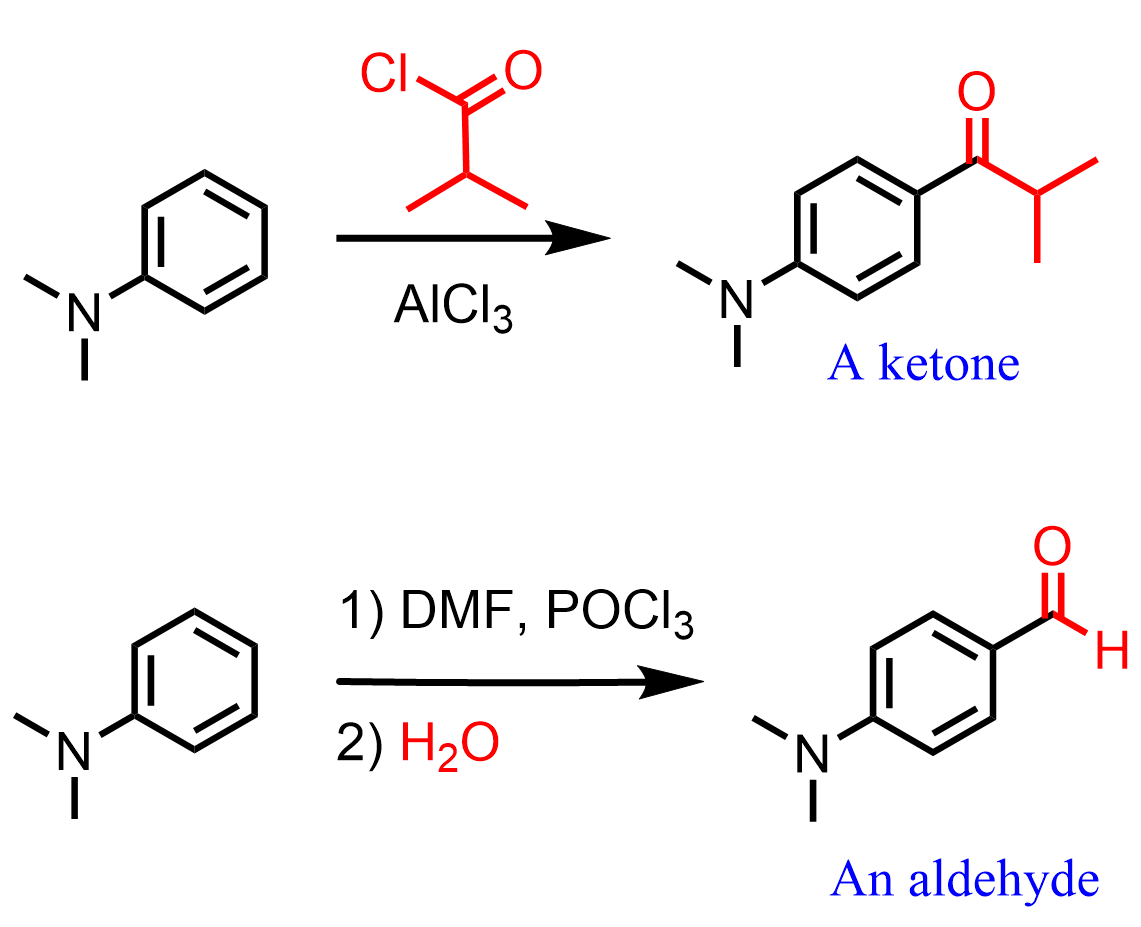
The mechanism and other specifics of this reaction are covered in a separate article, so feel free to check that out too.
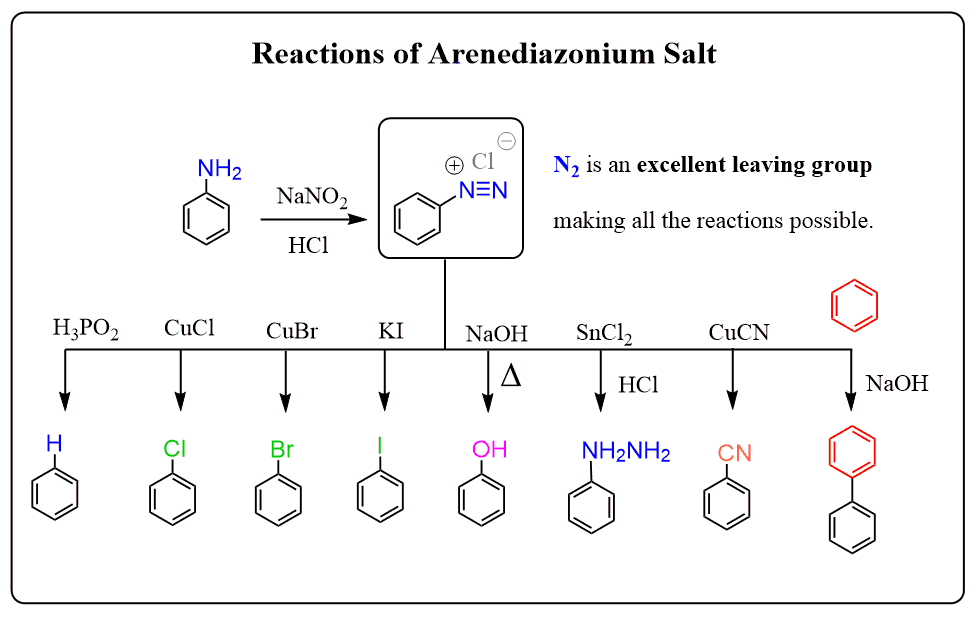
Aryl diazonium salts are prepared from aniline using nitrous acid at low temperatures:
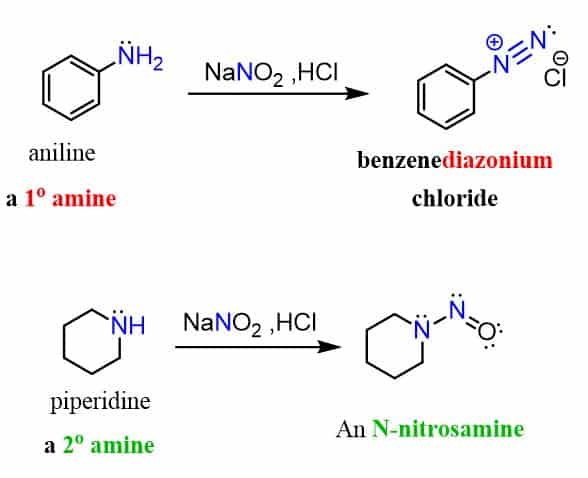
Arenediazonium salts represent an important tool in organic chemistry since they’re stable enough to be used as intermediates for preparing substituted benzene rings.
Below is the summary of the arene diazonium salt reactions, and more details can be found in this post:
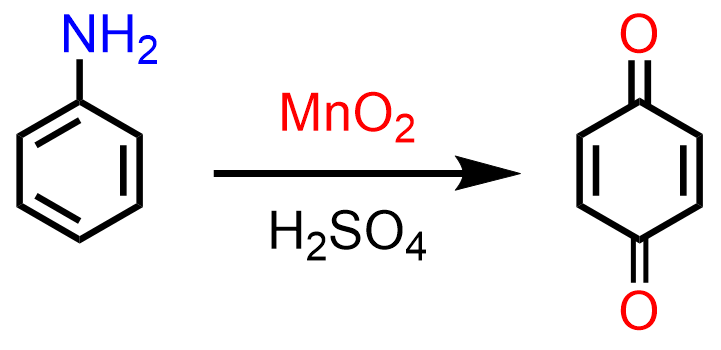
Aniline can be oxidized with manganese dioxide (manganese (IV) oxide) to benzo-1,4-quinone:
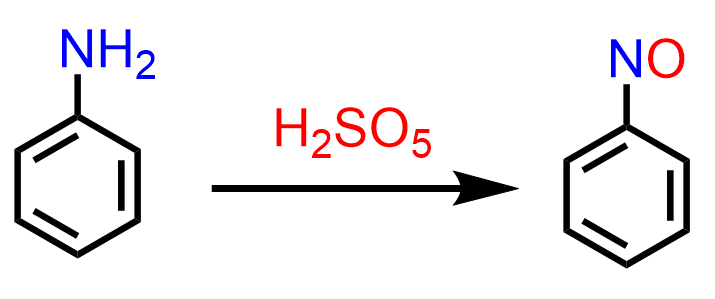
Nitrosobenzene can be prepared by the oxidation of aniline using peroxymonosulfuric acid (H2SO5):
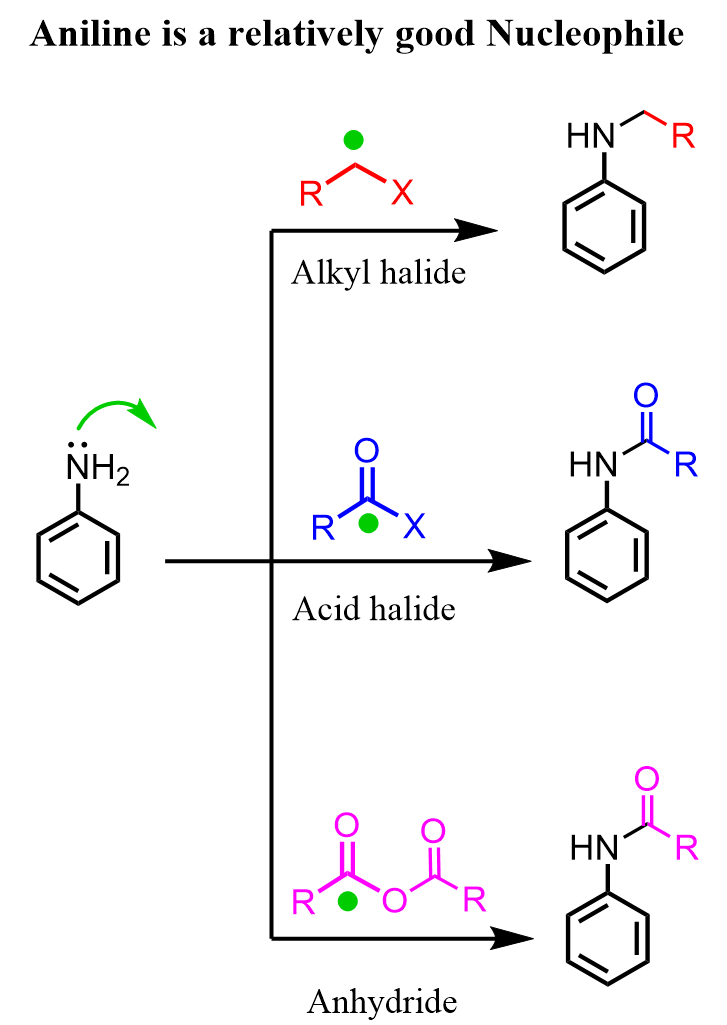
Even though the nitrogen of the NH2 group in aniline is not as nucleophilic as it is in aliphatic amines because of the delocalization of the lone pairs and the steric effect of the aromatic ring, it can still react with electrophiles such as alkyl halides, acyl halides, acid anhydrides, etc. We have seen a few times how the acylation of aniline is used to prepare a temporary protecting group. These reactions with anhydrides or acid chlorides occur via a nucleophilic addition-elimination mechanism, while the alkylation of the amino group can be achieved in a simple SN2 reaction:
Aniline derivatives also act as carbon nucleophiles due to the resonance activation of the ring. For example, arenediazonium salts, which are prepared from aniline, are great electrophiles due to the positively charged nitrogen, which pulls the electron density from the ring, making it susceptible to a nucleophilic attack. The reaction works best with highly activated benzene rings such as phenols, alkoxy benzenes, anilines, and N-alkyl anilines:
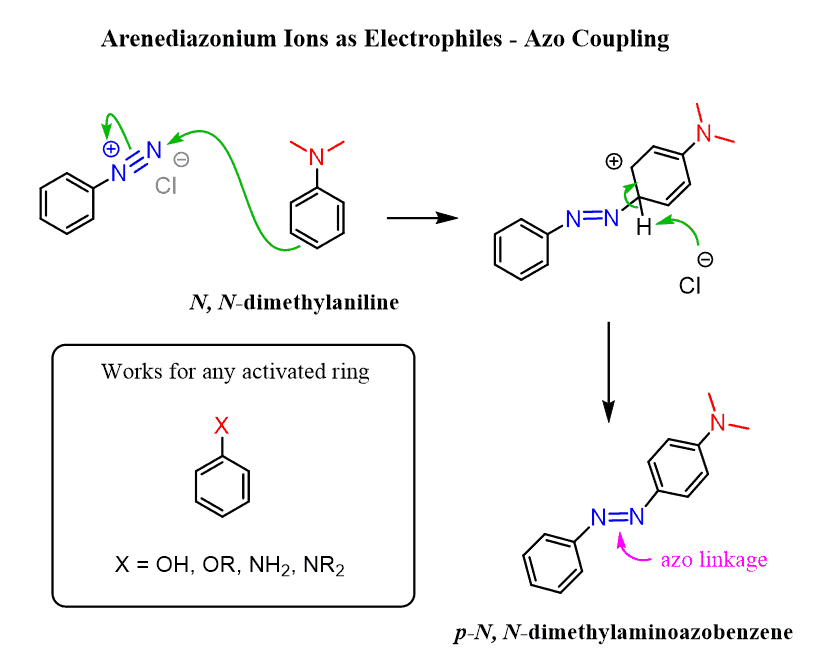
The product of the reaction is called an azo compound, which is colorful due to the extended conjugation. These chromophores are also known as azo dyes and are used to treat clothing, leather, and some foods.
